
Thomson found the particle to be negatively charged.He was also able to measure the charge-to-mass ratio of thecathode rays. He characterized the properties of cathoderays, as a stream of negatively charged particles orelectrons. Electron This was the first atomic particle discovered by J.J.

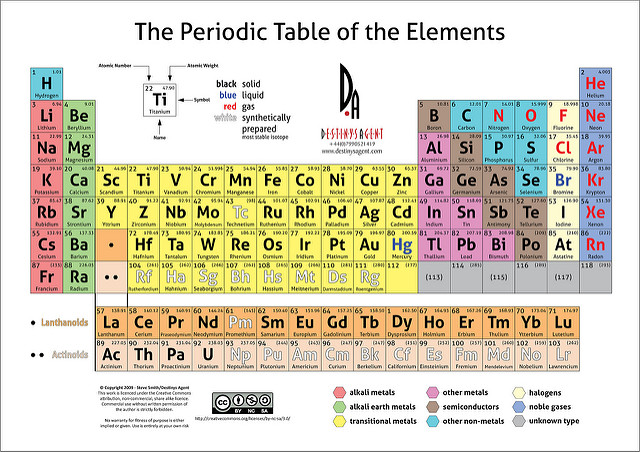
The electron is located outside the nucleus. The electron, the proton and the neutron.Protons and neutrons are together and make up the nucleus of anatom. Most of us know the three fundamental particles of which allatoms are composed. The metalsare blue and nonmetals are yellow. In the periodioc table below the metals, nonmetals andmetalloids are color coded for easy identification. The metalloids are a small collection of elements that liebetween the metals and the nonmetals in the periodic table andshare some of the properties of metals and nonmetals. are not malleable or ductile and are generally poor conductors (graphite is a very good electrical conductor). lack the remaining properties of the metals, i.e.are found in gas, liquid or solid state.
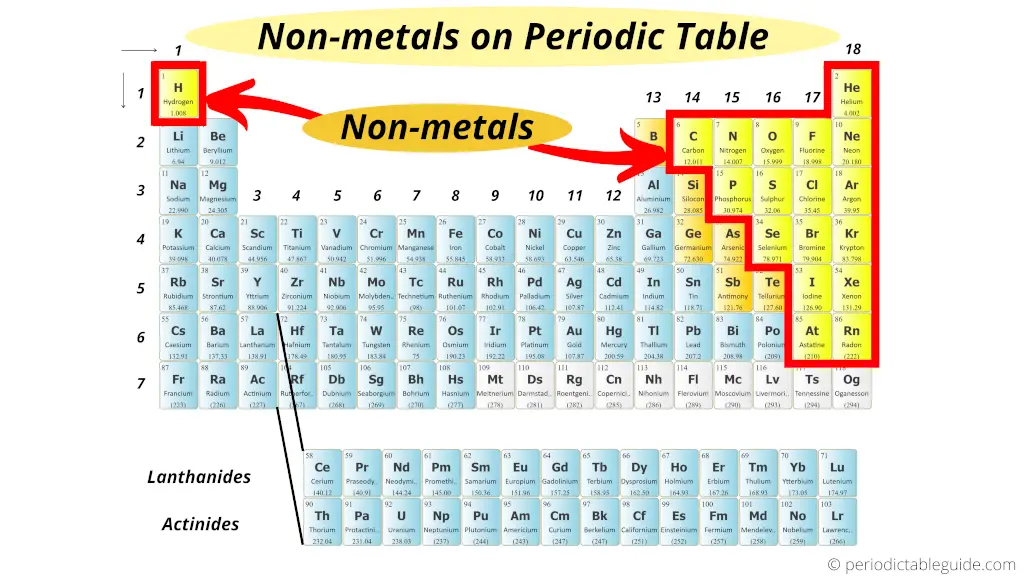
The next largest group are the nonmetals.


 0 kommentar(er)
0 kommentar(er)
